Case report feasibility of thoracoscopic epicardial pacing in two dogs
A three-port subxiphoid thoracoscopic approach was used. Pericardectomy was performed to access the epicardium. A sutureless unipolar epicardial pacing lead was placed. The lead was introduced through a 15 mm cannula positioned in the 6th left intercostal space and implanted within the epicardium. The pacemaker generator pocket was created between latissimus dorsi and intercostal muscles. Lead implantation was achieved after one attempt for dog 1, and three for dog 2. Surgical times were respectively 26 and 44 minutes. Pacemaker therapy was effective after surgery for both dogs. Complications included Twiddler’s syndrome in the first dog and a seroma in the second. The above technique was compared to the transdiaphragmatic approach in a limited cadaveric study on two dogs to establish the precise location of the epicardial leads for each technique. It demonstrated that thoracoscopic approach paced the right ventricle, while transdiaphragmatic technique paced the left ventricle.
http://dx.doi.org/10.1016/j.anicom.2017.08.001
2214-5672/© 2017 AFVAC. Published by Elsevier Masson SAS. All rights reserved
Faisabilité de la pose de pacemaker épicardique par thoracoscopie chez deux chiens : aspects clinique et chirurgical, et résultat
Résumé
Cet article rapporte la faisabilité de l’implantation épicardique de pacemaker par thoracoscopie chez deux chiens présentant une bradyarrythmie (bloc atrioventriculaire de grade 3), nécessitant la pose d’un pacemaker. Les examens complémentaires préopératoires ne montraient pas de contrindication à la pose d’un pacemaker. Un abord subxyphoïde à trois ports a été utilisé. Une péricardectomie était réalisée pour accéder à l’épicarde. Une sonde épicardique unipolaire sans suture était posée. La sonde était introduite dans la cannule de 15 mm positionnée dans le 6e espace intercostal et implantée dans l’épicarde. La poche du pacemaker était créée entre les muscles intercostaux et grand dorsal. Une tentative a suffi pour l’implantation pour le premier chien, contre trois pour le second. Les temps chirurgicaux étaient respectivement de 26 et 44 minutes. La stimulation cardiaque postopératoire était efficace chez les deux chiens. Les complications incluaient un syndrome de Twiddler chez le premier chien et un sérome chez le second. La technique thoracoscopique a été comparée à la transdiaphragmatique dans une étude cadavérique pour comparer la localisation de l’implantation. Celle-ci démontre que l’abord thoracoscopique implante le ventricule droit contrairement à l’abord transdiaphragmatique qui, comme attendu, implante le ventricule gauche.
Introduction
Artificial cardiac pacing in small animals treats symptomatic bradyarrythmias such as sick sinus syndrome, atrial standstill, and 2nd/3rd degree atrioventricular block (AVB) [1]. Currently, transvenous cardiac pacing (TV) is the main method used for cardiac pacing [1—4], but is not available in all veterinary hospitals. Epicardial pacing (EP) is the reference method for surgically placed cardiac pacing leads. It is performed in cases of unavailability, failure, infection or contraindication of TV (sepsis or pyoderma in the neck). Reported indications for choosing EP instead of TV are small dog size (mean weight: 5.3 ± 1.5 kg), concurrent abdominal surgeries (splenectomy, biopsies, cystotomy, tumor removal), diagnosis of valvular vegetative endocarditis, concurrent immunosuppression and arrhythmogenic right ventricular cardiomyopathy [5]. Surgical approaches for EP include left ventricle pacing via thoracotomy [6], median celiotomy and partial median sternotomy [7], transdiaphragmatic approach via abdominal incision [8] (TDA) and transxyphoid approach [9]. Surgical techniques allow direct visualization of cardiac structures and therefore correct lead positioning [10]. Thoracoscopic surgery is progressively gaining popularity as a minimally invasive approach, and its use is increasing in human medicine for EP [11,10]. The main benefits include reduced pain and quicker recovery.
The aim of this case report is to describe the feasibility of thoracoscopic EP (TEP), its advantages, associated risks, complications, and outcome. Additionally, a limited cadaveric study was performed to compare the location of lead implantation between thoracoscopic and transdiaphragmatic cardiac pacing.
Clinical report
Dogs recruited had symptomatic bradyarrythmias requiring pacemaker therapy. TV was not an option because of its unavailability. Complete data regarding each case were reviewed: age, sex, breed, history, clinical signs, general physical examination, cardiac examination, complementary examinations, surgical technique, surgical time, and followup. Cardiac examination included echocardiography and electrocardiography (Holter monitoring for 24 hours at rest) to investigate the bradyarrythmia, and to confirm the need for pacemaker therapy. Thoracic radiographs allowed for the exclusion of congestive heart failure, and Troponin-I testing permitted the exclusion of active myocarditis. Additionally, dogs underwent a complete blood count, biochemistry, lactate reading, and an abdominal ultrasound to exclude extra-cardiac causes of the arrhythmia.
Two dogs were recruited. Their characteristics are summarized in Table 1.
Surgery
Anesthesia
Intravenous premedication was performed using butorphanol (0.2 mg/kg intravenously [IV], Dolorex®, Intervet International, An Boxmeer, Netherlands) and midazolam (0.2 mg/kg IV, Midazolam, Panpharma, Fougeres, France). Alfaxolone (2 mg/kg IV, Alfaxan®, Jurox, Crawley, United Kingdom) was used for induction. Anesthesia was continued with isoflurane 2% (Vetflurane®, Virbac, Carros, France) and oxygen. Dogs received prophylactic antibiotic therapy (amoxicillin 20 mg/kg IV, Clamoxyl®, Glaxosmithkline, Marlyle-Roi, France). Once in the operating room, ventilation was controlled with intermittent positive pressure set at a tidal volume of 10 to 15 mL/kg and a respiratory rate of 10 to 12 breaths per minute. Anesthetic monitoring included pulse oximetry, non-invasive blood pressure, electrocardiography (EKG) and capnography.
Surgical preparation
Dogs were placed in dorsal recumbency. The entire thorax and abdomen (from shoulders to pubis 360◦) were clipped and prepared in aseptic fashion. This wide clip allowed for immediate conversion to open surgery (intercostal thoracotomy or trans-diaphragmatic approach) if necessary. A slight rotation to the right increased ease of approach for the surgical team by allowing more space for the implantation of the cannula on the left thoracic wall.
Approach
Three ports were used: one for telescope cannula and two for instrument cannulas (Fig. 1). A standard 5 mm 0-degree telescope (Hopkins® II straight forward telescope 0◦, Karl Storz Veterinary Endoscopy, Guyancourt, France) was introduced by a xyphoid portal in the right hemithorax (blue arrow, Fig. 1). An 11 mm cannula (trocar with pyramidal tip, Karl Storz Veterinary Endoscopy) with rotating insufflation stopcock was used to abolish pleural void by leaving it open.
The second port, 6 mm instrument cannula (trocar with pyramidal tip, Karl Storz Veterinary Endoscopy), was positioned in the 6th intercostal space on the right, opposite the chondrocostal junction close to the sternum (small orange arrow, Fig. 1). This port was used to introduce scissors or tissular fusion device (LigaSureTM, Covidien, Elancourt, France) to open wide the mediastinum and extend pneumothorax to the left hemithorax. A third cannula was inserted in the left 6th intercostal space. A wider cannula, 15 mm (VersaportTM Plus RPF 10—15 mm, Covidien), was chosen to minimize risk of pacemaker lead injuries during passage through the port (large orange arrow, Fig. 1).
Surgical technique
A pericardial window was performed as previously described [12]. An additional longitudinal incision, oriented to the left auricle, was needed to facilitate access to the ventricle [13]. A sutureless unipolar myocardial screw-in (pigtail) pacing lead for single chamber pacing was used (Medtronic Capsure® EPI 4965 coated with steroids, Medtronic Inc, Figure 1. Location of thoracoscopic portals: blue arrow = 11 mm telescope, small orange arrow = 6 mm right operating canal, large orange arrow = 15 mm left operating canal for insertion of the lead. Boulogne-Billancourt, France) (Fig. 2). The lead applicator was inserted through the left cannula with care to avoid stretching or twisting the lead during passage through the canal. Implantation was performed in the median portion of the ventricular epicardium in an area free of coronary vessels and away from the septum [13]. Screwing was performed during systole. A constant pressure was applied during screwing with care to avoid perforating the myocardium. Screwing was stopped after two turns as per the manufacturer recommendation. Before releasing the lead from its applicator, proper implantation was confirmed by careful traction applied on the lead. The cable was grasped with an endoscopic blunt grasper (Karl Storz Veterinary Endoscopy) and repositioned if needed, in order to make a loop oriented towards the cranial portion of the pleural cavity without twisting. This ended the thoracic phase of TEP. The lead exited the thorax by the penetration hole of the 15 mm cannula. A muscle pocket was dissected between intercostal and latissimus dorsi muscles. The pulse generator (Medtronic ADAPTA® ADSR01, Medtronic Inc) was connected to the lead and placed in its pocket to allow initialization of the cardiac pacing. The lead was allowed one loop within the pacemaker pocket to avoid any tension. The generator was programmed in VVIR mode (Ventricular pacing, Ventricular detection, Inhibition, Rate responsive) (Table 2). Cardiac frequency was set between 60 and 120 beats per minute (bpm), with inhibition rate set at 120 bpm.
A thoracic drain was temporarily placed to establish pleural vacuum after closure of the thorax wall. It was positioned in the right hemithorax to avoid interference with the cable. For this same reason, it was removed before complete awakening from anesthesia. The three portals were sutured in routine fashion.
Surgical time and perioperative complications were recorded (Table 2). No perioperative complications occurred during either procedure.
Post-operative management
Post-operative thoracic radiographs were performed to check for correct lead positioning (Fig. 3). Correct implantation implied that the lead must do one loop toward the cranial thorax and one within the pacemaker pocket to avoid any strain on the lead [13,5]. This was the case for both dogs. Lateral thoracic views suggested right ventricular implantation for both dogs. A postoperative echocardiography revealed no signs of hemodynamic abnormalities resulting from atrio-ventricular asynchrony [14,15] in either dogs. Both dogs were put on permanent EKG monitoring until discharge. Post-operative EKGs were satisfactory in both cases with stimulation spikes and ventricular depolarization following pacemaker stimulation. Cardiac frequencies were within the normal range (Table 2). EKG showed left heart delayed conduction suggesting that the right ventricle was stimulated first.
Dogs received a constant rate infusion of ketamine for 6 hours (4 g/kg/min IV, Kétamine® 1000, Virbac, Carros, France), along with morphine hydrochloride (0.2—0.3 mg/kg IV, Lavoisier, Paris, France) and a constant rate infusion of fentanyl (3 g/kg/h IV, Fentadon® 50 g/mL, Eurovet Animal Health, Bladel, Netherlands). This pain management was used to keep animals calm for proper cardiac monitoring.
Follow-up and outcome
Wounds and EKG check-up were performed 5 and 15 days after discharge. Relative exercise restriction was advised until wound healing. All medications were stopped for both dogs 15 days after discharge.
Case 1
The dog came back 19 days after surgery for reappearance of syncopes. There was no pacemaker capture on the EKG. A Twiddler’s syndrome was diagnosed with thoracic radiographs, which revealed coiling of the lead and pigtail head dislodgement (Fig. 4). Thoracoscopic reimplantation using the same surgical technique (TEP) was successfullyperformed. The lead was grasped with its applicator and screwed into the epicardium. Sutures were added between the epicardium and the pacemaker’s cuff. Pacemaker generator was changed one year and two years after first cardiac pacing. The dog died of unknown cause 4 years after cardiac pacing.
Case 2
A small subcutaneous seroma was present in the pacemaker pocket the day following surgery. It resolved itself within few days. The dog showed no other post-operative complications and had no more syncope after surgery. Pacemaker generator was changed 3 years after cardiac pacing.
Cadaveric study
A cadaveric study was performed on two medium breed dogs that were euthanized for reasons unrelated to the study. It was motivated by the fact that both post-operative EKG and radiographs suggested implantation of the right ventricle, whereas the heart was approached by the left hemithorax. TEP and TDA surgical approaches were achieved as previously described on both dogs. The epicardium was marked on the lead implantation site with a LigaSureTM vessel-sealing system (Covidien). Both hearts were then extracted from the thorax and fully dissected to localize the site of epicardial pacing to the right or left ventricle. They showed that the right ventricle was paced with TEP, and the left ventricle was paced by TDA, both sites of pacing being close to the interventricular septum (Fig. 5).
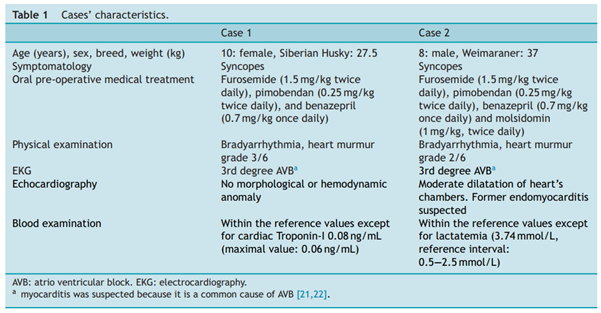
Table 1
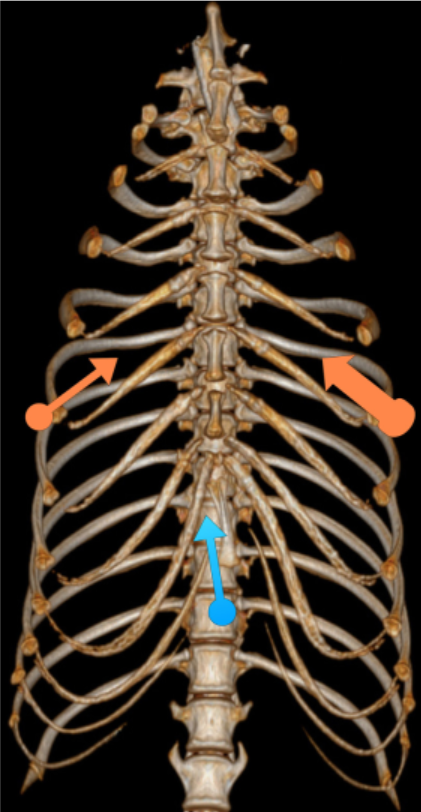
Figure 1
Location of thoracoscopic portals: blue arrow = 11 mm telescope, small orange arrow = 6 mm right operating canal, large orange arrow = 15 mm left operating canal for insertion of the lead.
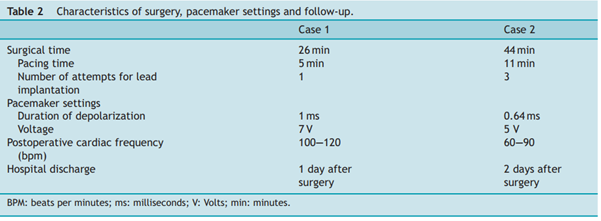
Table 2
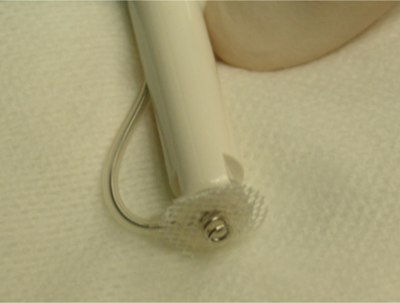
Figure 2
Sutureless unipolar myocardial screw-in pacing lead.
Note the pigtail fashion that allows implantation and maintenance of the lead within the epicardium. A fibrosis between the white patch and the epicardium will develop and strengthen the adherence between the lead and the epicardium.
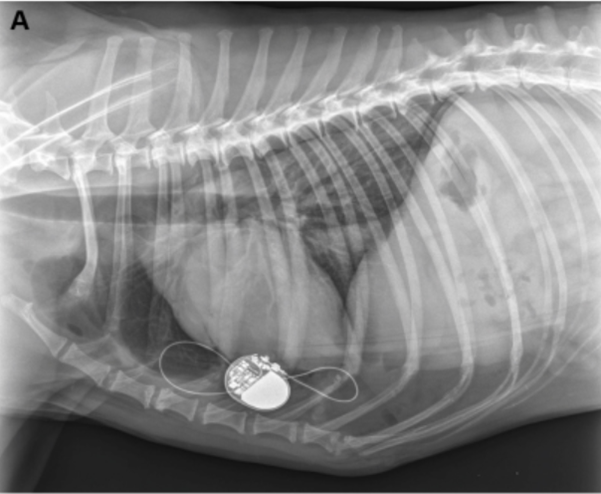
Figure 3a
Post-operative thoracic radiograph (right lateral view) confirming good position of the pacemaker lead in both cases (3A is case 1, 3B is case 2).
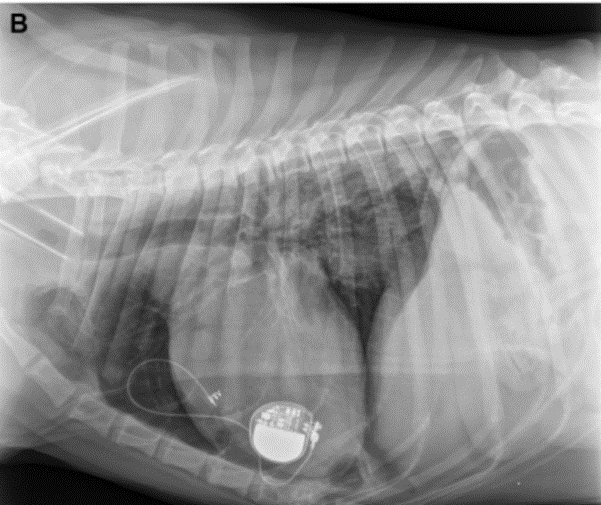
Figure 3b
The lead makes one loop in the thorax, and one in the pacemaker pocket. This view suggests that the lead paces the right ventricle.
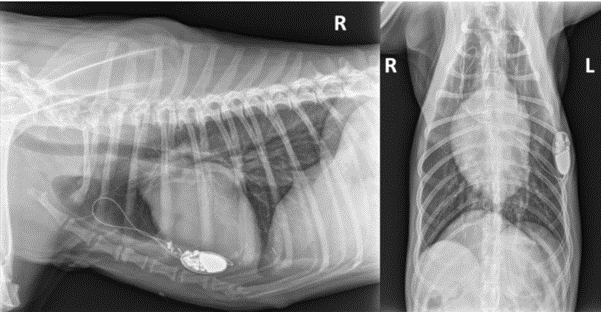
Figure 4
Thoracic radiographs (right lateral and ventro-dorsal views) showing lead coiling and dislodgement (Twiddler’s syndrome) in case 1.
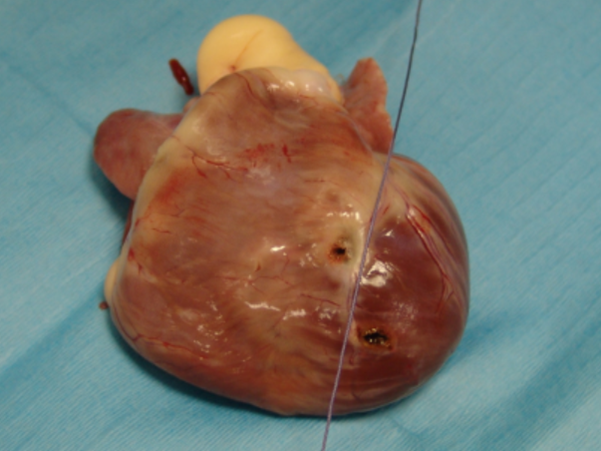
Figure 5
Cadaveric study. Appearance of the heart after extraction from the thorax and partial dissection. The string represents the inter-ventricular septum. The right ventricle is on the left side of the string, the left ventricle is at its right. Each hole represents the location of implantation of the lead. Right ventricle for TEP, and left ventricle for TDA.