Abstract
Objective To evaluate anaesthetic death after implementation of recommendations and its risk factors in a small animal practice.
Study design Observational cohort study.
Animals All cats and dogs anaesthetized at the Centre Hospitalier Vétérinaire des Cordeliers during two periods, from April 15th, 2008 to April 15th, 2010 (period 1) and from June 15th, 2010 to August 24th, 2011 (period 2).
Methods Death occurring during or before full recovery from anaesthesia was recorded. At the end of period 1, a logistic regression model was generated to describe anaesthetic death and identify risk factors. Potential risk factors in our practice setting were identified, and three recommendations, relating to improving physical status and anaesthetic/analgesic regimen implemented for period 2. The relationship between anaesthetic death and recorded variables were analyzed, and where relevant, compared between periods.
Results Six thousand two hundred and thirty-one animals underwent general anaesthesia. The overall death rate during period 1 was 1.35% (48 in 3546, 95% CI [1.0–1.7%]) and during period 2 was 0.8% (21 in 2685, 95% CI [0.6–1.2%]). For sick animals (ASA status 3 and over), the overall death rate was 4.8% (45 of 944 95% [CI 3.5–6.4%]) during period 1 and 2.2% (18 of 834 95% CI [1.3–3.5%]) during period 2; this represented a significant decrease in death rate in period 2 (p = 0.002). In period 2, the main factors associated with an increased odds ratio of anaesthetic death were poor health status (ASA physical status classification) and old age. Species, gender, anaesthetic regimen, the nature and urgency of the procedure were not associated with risk.
Conclusion and clinical relevance Following evidence based recommendations, the death rate related to anaesthesia was significantly decreased during period 2 compared to period 1. Application of evidence-based medicine may contribute to an effective approach to decrease death rates. Other factors, not monitored in this study, may also have had an impact.
Keywords anaesthetic death, cat, dog, evidencebased medicine, mortality, risk, small animal.
Introduction
Evidence-based medicine (EBM) has been defined as the ‘conscientious, explicit and judicious use of current best evidence in making decisions about individual patients’ (Sackett et al. 1996). An extension is evidence-based veterinary medicine (EBVM). Its philosophy has been described as ‘using the best available evidence and your clinical expertise to make the best clinical decisions for your patients and clients’ (Schmidt 2007). The practice of EBVM involves a 5-step approach (Robertson 2007; Schmidt 2007).
- Convert information needs into answerable questions.
- Efficiently track down the best evidence to answer the question.
- Critically appraise the evidence for its validity and usefulness.
- Integrate appraisal results with clinical expertise and patient values.
- Evaluate outcomes.
Our team has applied the first three steps of EBVM to anaesthetic death in small animal practice (Bille et al. 2012).
- Answerable questions judged to be of clinical importance were: what is the anaesthetic death rate in small animal practice? Are there any identified risk factors?
- The search for the best evidence yielded numerous results. In the last 25 years, the risk of anaesthetic death has been studied in dogs and cats by teams in different countries (Clarke & Hall 1990; Dodman & Lamb 1992; Dyson et al. 1998; Hosgood & Scholl 1998, 2002; Gaynor et al. 1999; Joubert 2000; Williams et al. 2002; Brodbelt et al. 2006, 2007, 2008a,b; Redondo et al. 2007; Alef et al. 2008; Bille et al. 2012; Gil & Redondo 2013).
- Of these, studies that included logistic regression models to analyse risk factors for anaesthetic death in small animal practice were considered to be the best suited to answer both of our questions (Dyson et al. 1998; Hosgood & Scholl 1998, 2002; Brodbelt et al. 2006, 2007, 2008b; Bille et al. 2012; Gil & Redondo 2013). These models allow veterinarians readily to identify risk factors for anaesthetic death.
To the authors’ knowledge, no published paper has described the application of the fourth and fifth steps to anaesthetic death in small animal practice.
The authors conducted a first cohort study at the Centre Hospitalier Vétérinaire des Cordeliers during a 2-year period in order to evaluate the anaesthetic death rate in their practice and used a logistic regression model (Appendix S1) to describe it (Bille et al. 2012). The study demonstrated that the main factor associated with increased odds of anaesthetic related death was poor health status [as categorized using the Association of American Anesthesiologists (ASA) physical status scale]. However there was a tendency for regimens using a combination of a premedication agent, induction with thiopental and maintenance with isoflurane to increase the odds of death compared with other anaesthetic induction techniques, and also a tendency for increased odds of death in dogs receiving epidural analgesia. Application of this evidence together with our clinical expertise led senior staff members to make the following recommendations.
- Emphasize pre-anaesthetic medical management of the patient whenever possible, in order to improve patient’s clinical (ASA) status before inducing anaesthesia.
- For patients of ASA status 3 and over, preferably use a regimen including a premedication, anaesthetic induction by intra-venous (IV) administration of propofol and maintenance by isoflurane.
- Limit the use of the combined epidural injection of morphine and bupivacaine.
The aim of this current study was to evaluate anaesthetic death rate after implementation of the above set of recommendations and to re-evaluate the risk factors now prevalent. The authors hypothesized that the death rate in their practice would decrease significantly.
Methods
We used an observational cohort study design.
Patient recruitment
Patients were recruited over two periods (Fig. 1). Period 1 (Bille et al. 2012) was from April 15th 2008 to April 15th 2010. Following the findings of Period 1 (Appendix S1) senior staff members released their three recommendations to all attending veterinarians in our facility. The recommendations were explained verbally and posted on the induction room wall: A 1-month acclimatization period was allowed between the time recommendations were given and the beginning of the second period of data collection. Period 2 covered anaesthetics given from June 15th 2010 to August 24th 2011.
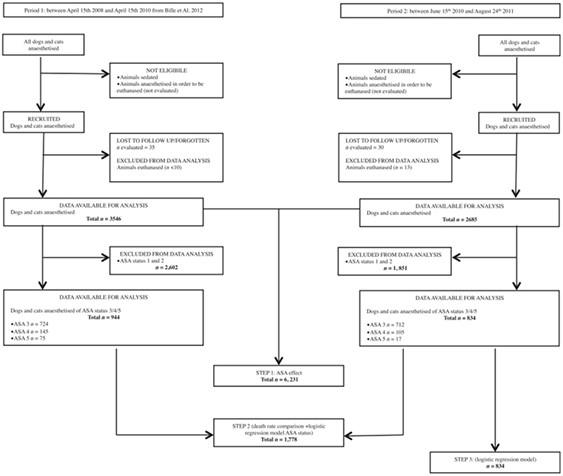
Fig. 1 – Flow diagram of the recruitment and follow-up of the anaesthetic cases.
All dogs and cats that underwent general anaesthesia at the Centre Hospitalier Vétérinaire des Cordeliers during period 1 and period 2 were included. General anaesthesia was defined as a druginduced unconsciousness characterized by a controlled and reversible depression of the central nervous system (CNS) sufficient to allow endotracheal intubation if required. Animals that were sedated or anaesthetized in order to be euthanazed were not eligible. Sedation was defined as chemical restraint insufficient to allow endotracheal intubation.
The patients’ inclusion commenced when general anaesthesia was induced by administration of an injectable induction agent, by whatever route [IV or intramuscular (IM)], or isoflurane until the end of anaesthesia. End of anaesthesia was defined as the return of consciousness characterized by the observation of the patient interacting with its surroundings, rectal temperature above 36°C and sternal recumbency. Animals that were euthanazed during anaesthesia for medical reasons were excluded from the study.
For both periods, death occurring during anaesthesia was recorded.
Data collection
For all patients that were included, identification data, species, age, type of procedure, anaesthetic regimen and epidural administration of a combination of morphine and bupivacaine were recorded.
Prior to induction of anaesthesia, on the basis of history and clinical examination, the attending anaesthetist assessed and recorded the patient’s ASA status. The anaesthetist then decided which anaesthetic regimen would be used.
Following completion of period 1, authors considered that not all available relevant data had been taken into account. Therefore, during period 2, patients’ breed, gender, weight and urgency of the procedure were also recorded.
Grouping
Grouping of patients recruited during period 1 has been described elsewhere (Bille et al. 2012). For patients recruited during period 2, some groupings differed from those described in period 1.
In period 1 animals were grouped according to their actual age. However, in period 2 the animals were grouped in order to reflect the age in relation to the expected life-span of their breed. (Moreau et al. 2003; Adams et al. 2010). The animals’ pedigree papers identified breeds. Animals that were older than the median age at death described for its category were arbitrarily classified as ‘geriatric’. For crossbred dogs, median age at death was considered to be median age at death of the overall population (11.25 years). For cats, a median age at death of 12 years old was retained.
The type of procedure the patient underwent was defined as: ‘examination’ (a non-invasive procedure), ‘orthopaedic’ or ‘soft tissue surgery’. No animal died in the ‘orthopaedic’ category; therefore, ‘orthopaedic’ and ‘soft tissue surgery’ categories were merged into a single ‘surgery’ category (any invasive procedure). If an anaesthetized animal underwent more than one procedure, the most invasive one was retained for the study.
Premedication, anaesthetic induction and maintenance agents were recorded. Twenty-two different premedication regimens were identified. They included the use of acepromazine, midazolam, morphine, fentanyl, butorphanol and medetomidine, used either alone or in combination. For the data analysis, all premedication regimens were gathered in a ‘Yes’ category. The nature of the substance used and the route of administration were not taken into account. Any patient that did not receive premedication was included in a ‘No’ category.
Thirteen different induction regimens were identified. They were grouped into two categories: ‘Propofol’ and ‘alternative induction’. The latter included the use of ketamine, thiopental, fentanyl, isoflurane, medetomidine or dexmedetomidine or its use with ketamine, propofol or thiopental. Two groups of maintenance regimens were defined: patients that received isoflurane and patients that did not.
Patients that did not receive isoflurane were anaesthetized by means of total intravenous anaesthesia or the IM injection of the induction agent with no maintenance.
The anaesthetic regimen was defined by the combination of the premedication, the induction and the maintenance regimens. One hundred and sixteen different anaesthetic regimens were identified. Two main groups were defined:
- Use of the recommended protocol (premedication, induction of anaesthesia with propofol, maintenance with isoflurane).
- Use of an alternative anaesthetic regimen. This group included three subgroups:
- premedication and induction with thiopental, maintenance with isoflurane.
- premedication and induction with ketamine, maintenance with isoflurane.
- other (any other combination).
Some variables were only present in period 2. A study by Brodbelt et al. (2008b), found that a bodyweight of <5 kg was a risk factor for anaesthetic-related death in dogs. In period 2 of this current study animals were grouped into three categories: ‘≤5 kg’, ‘>5 kg’ and ‘not weighed’.
The urgency of the procedure was also assessed only in period 2. A procedure was classified as ‘Scheduled’ if staff were notified at least four hours prior to the procedure; as ‘Urgent’ if it was not ‘Scheduled’ but medical management was possible before anaesthesia was induced and as ‘Emergency’ if it was not ‘Scheduled’ and medical management was not possible before anaesthesia was induced.
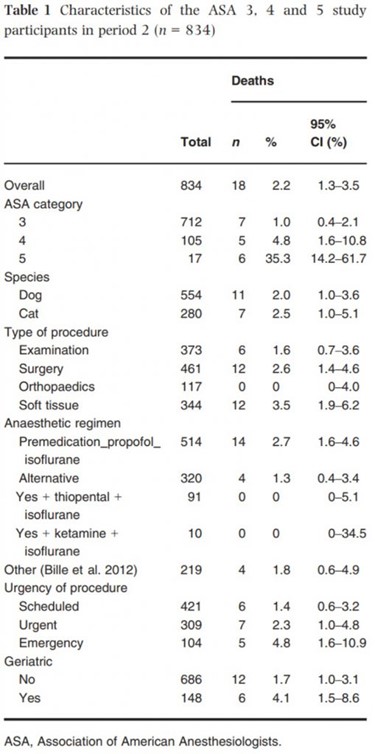
The characteristics of ASA 3, 4 and 5 study participants during period 2 are shown in Table 1. The Centre Hospitalier Vétérinaire des Cordeliers acquired a computed tomography scanner (CT) in December 2009. Details of animals undergoing CT scans were specifically noted.
Bias assessment
To limit inter-observer consistency of the ASA classification the same attending veterinarian (CB) reviewed and, if necessary, reassessed the ASA status on the basis of the patient charts.
The authors aimed to include 950 patients with an ASA status of 3 and over for period 2, in order to reach the same number as in period 1 and allow statistical comparison. However, during the study the sale of the formulation of propofol used in Study 1 was discontinued in France, therefore data collection for the study was terminated on August 24th 2011.
Data analysis
If an animal underwent more than one anaesthesia, each event was considered independently.
The data analysis was conducted following a three-step approach. As a first step, the relationship between death and ASA score was studied. As a second step, because of the very low death rate in animals with an ASA score of 1 or 2 (see Results), the analysis focused on animals with an ASA score of 3 and over. Univariate analysis was performed to compare the characteristic of the two cohorts (Chi square and Mann–Whitney/Wilcoxon Two-Sample Test, EpiInfo 3.5.3; Centers for Disease Control and Prevention, GA, USA). Finally, as a third step, as more variables were available for period 2 than for period 1, a specific analysis was performed on patients with an ASA status of 3 and over in period 2. Univariate analyses were carried out, followed by a multivariate analysis of the data. The statistical unit was the animal and death the outcome variable.
Logistic regression models were created using the GLM procedure of R software 2.13.1 (R Foundation for Statistical Computing, Austria). ‘Period’, ‘ASA category’, ‘species’, ‘type of procedure’, ‘anaesthetic regimen’, ‘urgency of procedure’ and ‘geriatric’ were set as explanatory variables. The probability of wrongly rejecting the null hypothesis was set to 5%. A manual backward procedure was used.
Variables were kept in the model if p < 0.16 and therefore tested for collinearity performing chisquares. Two-way interactions were tested. For assessing the fit of the models, the le Cessie-van Houwelingen-Copas-Hosmer global goodness of fit test were calculated, receiver-operating characteristic (ROC) curves were constructed and the areas under curves (AUCs) were calculated (R software, MKmisc, PresenceAbsence and RelativeRisk libraries).
Animals with missing data (n = 60) were excluded (Fig. 1). This included only animals for which age was missing. The physical status of these animals were ASA 1 (n = 26), ASA 2 (n = 23), ASA3 (n = 10) or ASA4 (n = 1). None of them died during anaesthesia.
Results
Participants and descriptive data
The duration of period 2 (14.3 months) was shorterthan that of period 1 (23.8 months). During the study periods, 6231 animals underwent general anaesthesia; 3546 patients were recruited during period 1 and 2685 during period 2. When considering patients with an ASA status of 3 and over, 944 animals were recruited during period 1 and 834 during period 2. Animals lost to follow-up were estimated to be 60 (30 per period). The number of patients euthanized for medical reasons during general anaesthesia was estimated at <10 during period 1 and was recorded as 13 during period 2 (Fig. 1).
Outcome data
During period 1, 48 animals out of 3546 (1.4%, 95% confidence interval (CI) [1.0–1.7]) died. During period 2, 21 animals out of 2685 (0.8%, 95% CI [0.5–1.2%]) died. No animal died in the ASA 1 category. The death rate increased with each grade of ASA status (Tables 1 and 3).
Main data
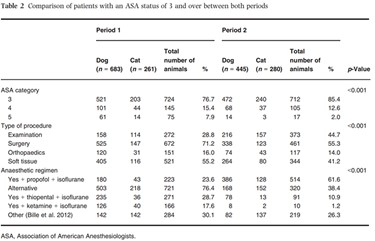
Patients with an ASA status of 3 and over from both periods were compared. Characteristics of the ASA 3, 4 and 5 study participants during both periods are presented in Table 2. There were significantly more cats (p = 0.008) in period 2 (33.6%) than in period 1 (27.6%). ASA status, anaesthetic regimen and type of procedure distributions differed significantly between periods.
During period 1, 45 patients with a status of 3 and over [4.8%, 95% CI (3.5–6.4%)] died under anaesthesia, while during period 2, 18 [2.2%, 95% CI (1.3–3.5%)] died under anaesthesia (Table 2). For these patients, the death rate was significantly lower (p = 0.002) during period 2.
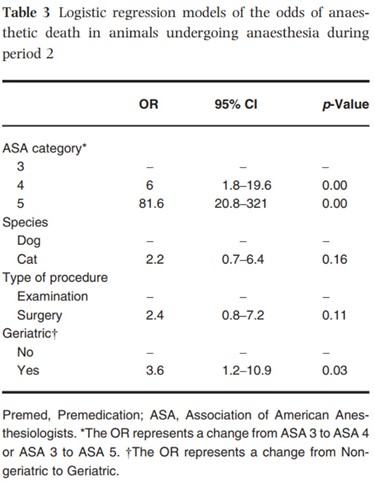
The third-step (multivariate) analysis was performed on the ASA 3, 4 and 5 categories during period 2 (Table 3). No co-linearity was detected between the variables of the model. Dogs had surgery significantly more often than cats but the association between the variables ‘type of procedure’ and ‘Species’ was not strong enough to interfere with the model. A higher ASA status and being geriatric were associated significantly with an increased likelihood of anaesthetic death. Increasing ASA category was very significantly associated with an increased chance of death, and compared to the group ‘Not geriatric’, the group ‘Geriatric’ was associated with a significant 3.6-fold increase in the chance of death. Although not statistically significant, there was a tendency for cats to be more at risk of death during anaesthesia than dogs, and for animals undergoing surgery to be more at risk than those just undergoing examination. After adjusting for other variables, the odds of death associated with patients’ breed, gender, the anaesthetic regimen or urgency of the procedure were not significantly different. The le Cessie-van Houwelingen-Copas-Hosmer global goodness of fit test p value was 0.63. None of the interaction terms were significant and were therefore dropped out of the model. The AUC of the model was 0.80, indicating good discrimination properties (Hosmer & Lemeshow 2000).
Analyses of subgroups and interactions
Characteristics of ‘CT scan’ subgroup are presented in Appendix S2. During period 1, 110 CT scans were performed compared to 453 during period 2. Only those performed on patients of ASA 3, 4 and 5 categories were analyzed. This represented 24 procedures during period 1 and 128 during period 2. Of these, 17 were classified as ‘examination’ in period 1 and 113 in period 2. CT scans represented 6.25% of all ‘examination’ procedures in period 1 and 30.3% in period 2.