Evaluation of coexisting polymyositis in feline myasthenia gravis : A case series
Abstract
Acquired myasthenia gravis (MG) is relatively uncommon in cats. In humans, MG may be associated with other immune-mediated disorders, in particular polymyositis (PM).
In this study, we described in-depth electrodiagnostic findings and pathological changes in muscles of cats diagnosed with MG, and assessed the presence of concurrent PM.
Six cats with confirmed acetylcholine receptor antibody seropositive MG, and two suspected cases with clinical signs and electrophysiological changes consistent with MG, were reviewed.
All animals presented with severe typical signs of generalized weakness and/or fatigability, resembling late-onset MG in humans, in addition to regurgitation.
Five cats presented a cranial mediastinal mass, with 3 confirmed as thymoma.
Repetitive nerve stimulation revealed a decrement of the compound muscle action potential in all tested cases, starting from low frequencies of stimulation.
Serum creatine kinase activity was increased in 6/8 cats. Muscle biopsies performed in 5 cats revealed varying degrees of mixed mononuclear cell infiltrates, positive for the leukocyte markers CD3/CD4/CD8 and CD11b.
Further MHC-1/C5b-9 positive sarcolemmal deposits were identified in all tested cases, with or without thymoma.
This study documents an association of MG and PM in cats, and provides further support for feline MG as a relevant animal model of human MG.
© 2017 Elsevier B.V. All rights reserved.
Introduction
Acquired myasthenia gravis (MG) is an immune-mediated disease affecting the neuromuscular junction (NMJ). Acquired MG is characterized by destruction and loss of acetylcholine receptors (AChR) on the motor end-plates of striated muscles by autoantibodies, delaying neuromuscular transmission and resulting in muscle weakness [1]. The most common clinical presentations in feline MG include generalized weakness, ventroflexion of the neck and a cranial mediastinal mass [2, 3].
The first report of feline MG was in 1970 [4]. Since then, several case reports and a few case series have contributed to our knowledge of this disease in cats [5-11]. Two extensive studies [2, 3] confirmed risk factors for feline MG in 340 cats with a diagnosis of MG based on positive AChR antibody titers (seropositive MG).
These studies identified the most common clinical presentations and documented the association of a large percentage of feline MG cases (52%) with thymoma. However, in-depth studies of electrodiagnostics and muscle pathology were lacking in these previous reports.
In humans, MG has been associated with other immunemediated muscle diseases, such as polymyositis (PM), dermatomyositis (DM) [12], and granulomatous myositis [13]. An association with thymoma has been found in 10 to 30% of human cases, depending on the racial type and MG form [14].
In one publication reporting a case of feline concurrent polyneuritis and PM [15], the authors briefly discussed biopsyconfirmed PM in 17 cats, with 2 cases of concurrent MG and thymoma. To the author’s knowledge, PM associated with feline MG has only been described in a single published case report [11].
PM, in the absence of MG, can also be associated with thymoma in humans and cats [12, 16] .
The objectives of this study were to review clinical cases of feline MG presented at a veterinary teaching hospital and clinical practices in France, to better describe the electrophysiological characteristics of feline MG (with focus on repetitive nerve stimulations), to search for indicators of concurrent polymyositis, and to compare these features with human MG.
Materials and methods
Case selection
Inclusion criteria comprised a detailed medical record, clinical signs consistent with MG, a positive AChR antibody titer performed by species-specific immunoprecipitation radioimmunoassay at the Comparative Neuromuscular Laboratory, University of California, San Diego, and an electrophysiological examination.
Data collected from medical records (from software CLOVIS,4Dv13) included signalment, history, results of physical and neurological examination, pharmacologic testing, serum biochemistry panel and complete blood count, infectious diseases screening including testing for feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV), thoracic radiographs, fluoroscopy, ultrasonography or CT, electrophysiological features, including repetitive nerve stimulation (RNS), and muscle biopsies. Information about the treatments and outcome was also collected.
All clinical examinations were conducted by a board-certified veterinary neurologist or a resident in neurology.
Pharmacological testing
A videotape of the gait and exercise tolerance was made prior to pharmacological testing. Neostigmine methylsulfate (20–30 g/kg, Prostigmine, Meda Pharma, France) was administered intravenously when exercise was no longer possible.
The clinical response to the anticholinesterase drug was evaluated and recorded for 15 to 20 minutes after administration. Resolution of or at least a strong improvement in clinical weakness was considered as a positive test.
Parasympathetic adverse effects were evaluated by monitoring the heart rate, pupil diameter, and occurrence of diarrhea or abundant ptyalism. In the case of adverse effects, 20–30 g/kg of atropine was immediately administered intravenously.
Electrodiagnostic testing
Electrophysiological procedures were conducted under general anesthesia. The anesthetic protocol consisted of intravenous administration of 4–6 mg/kg propofol, maintained by inhalation of isoflurane (2–3%) mixed with oxygen. NaCl 0.9% was administered at 8 mL/kg/h in IV perfusion throughout the procedure.
The animal was placed in right lateral recumbency, so that all the electrophysiological examinations could be performed on the left side. The cat’s temperature was maintained between 37 and 38 °C using a heating mat.
All examinations were performed by a board-certified neurologist or resident in training, using the same procedure and the same electrodiagnostic device.1
A disposable bipolar concentric needle electrode was used for electromyography (40 mm length, 0.45 mm width, needle, with a 0.068 mm2 sampling area). Appendicular, paraspinal and head muscles were tested. Prolonged insertional and abnormal spontaneous electrical activity were recorded and graded (mild, moderate, or marked).
In the motor and sensory nerve conduction studies, polytetrafluoroethylene-coated stainless steel monopolar electrodes of different lengths with 3 mm bare tips were used for stimulation and recording. Compound muscle action potentials (CMAP) were obtained with supramaximal stimuli of 0.1 ms duration, delivered at a frequency of 1.5 hertz (Hz). For each animal, the left tibial, peroneal, ulnar and radial nerves were tested.
Repetitive nerve stimulations of the peroneal nerve were performed using adhesive recording surface electrodes, after clipping the area of the left tibialis cranialis muscle. Supramaximal repetitive stimulation was first delivered at a rate of 0.1 Hz, to test the stability of the device, and then increased gradually from 0.2 to 10 Hz (with trains of 10 stimuli for each stimulation rate).
The recovery time between two stimulation trains was at least 2 minutes. The presence of a decremental response was assessed for each stimulation rate, by comparing the CMAP amplitudes with the first measurement. A decremental response was defined as positive if the initial CMAP amplitude was decreased by more than 10%.
RNSs were repeated with the same parameters 10 to 20 minutes after an intravenous neostigmine methylsulfate administration (20–25 g/kg).
All electrophysiological parameters obtained were compared with previously published data in the cat [17] , as well as with 2 healthy control cats for the RNS studies.
1 Nicolet Viking Select & Nicolet Viking IV P, Viasys Healthcare, Madison, Wisconsin.
Muscle biopsies and histological examination
Muscle biopsies were performed under general anesthesia on the animal’s right side, immediately after the electrodiagnostic procedures.
The skin on the areas of interest was clipped and surgically cleaned. Prophylactic antibiotics (cephalexin or amoxicillin), morphine (0.2 mg/kg IV), and meloxicam (0.1 mg/kg subcutaneously) were administered prior to the procedures. Biopsies were collected from the triceps brachii, biceps femoris and tibialis cranialis muscles by an open procedure, then immediately frozen in isopentane cooled in liquid nitrogen (−130 °C), and stored at −80 °C until processed. Transverse cryosections (10 m thick) were stained using standard protocols, including hematoxylin–eosin (H&E) and modified Gomori trichrome (TG) stains.
Inflammatory cell infiltration and major histocompatibility complex class 1 (MHC-I) or complement expression were immunohistochemically characterized by processing transverse cryosections (7 m thick) with an EnVision Detection Systems Peroxidase/DAB Kit (Dako) using the following antibodies: primary rabbit monoclonal antibodies against feline CD3 cell surface antigen, expressed by mature T lymphocytes (1:200, ABCam, United Kingdom), mouse monoclonal antibody against feline CD4 cell surface antigen, expressed by T4 lymphocytes (1:500, ABCam), rabbit polyclonal antibody against feline CD8 cell surface antigen, expressed by T8 lymphocytes (1:500, ABCam), mouse monoclonal antibody against feline CD11b cell surface antigen, expressed by granulocytes, monocytes and some macrophages (1:50, AbD Serotec), mouse monoclonal antibody against MHC-I, (1:300, Linaris) and mouse monoclonal antibody against anti-Human C5b-9 activated complex (1:100, Linaris).
Each section was compared to a negative control (healthy cat) from the neurology unit database, as well to a positive control (popliteal lymph node from a healthy cat).
Histochemical localization of acetylcholine esterase (AChE) at the motor end-plates was performed using the esterase reaction (Karnovsky and Roots reaction).
Fluorescent localization of AChR at the motor end-plates was performed by incubating sections with Alexa Fluor 488 α-bungarotoxin (α-bgt, 1:1000, Life Technologies) for 30 minutes. The esterase reaction and α-bgt stainings were done to precisely localize the motor end-plates and to compare them with the distribution of inflammatory cell infiltrates.
Polymyositis was defined, in addition to elevated serum CK activity and consistent electrophysiological abnormalities, by the presence of inflammatory cell infiltrates with H&E, and IHC, and/or sarcolemmal expression of MHC-I or C5b-9 in muscle samples [18].
PM was then graded as mild (+), moderate (++) or marked (+++), and defined as follows: mild, rare and sparse inflammatory cell infiltrates; moderate, several sustained multifocal area of inflammatory cell infiltrates; and marked, diffuse extensive inflammatory cell infiltrates.
Results
Animals and history
Six cats (cases 1 to 6) followed up between 2008 and 2015 after a definitive diagnosis of MG met the inclusion criteria. Two additional cats (cases 7 and 8) were also included but were treated separately.
Cases 7 and 8 both presented with clinical signs of weakness and electrophysiological changes consistent with MG. Case 7 had a thymoma and high serum CK activity. The AChR antibody titers were either not measured (case 7) or were within the reference range (case 8).
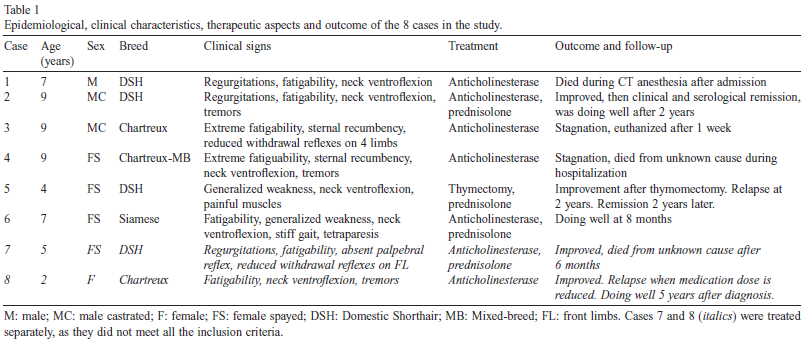
Signalments at the time of presentation are shown in Table 1. Three cats were male (1 intact, 2 castrated) and five were female (1 intact, 4 spayed). The median age was 7 years. Breeds included four domestic shorthair (DSH), three Chartreux (including one mixed-breed cat), and one Siamese cat.
All cats had exhibited a recent onset of clinical signs, ranging from one week to two months. The most frequent reasons for presentation were difficulties in ambulation and reluctance to move (7/8), weakness (4/8), regurgitation (4/8), tremors (3/8), and dysorexia (3/8).
None of the cats received any medication at the time of presentation with the exception of one cat (case 2), which had received corticosteroids and antibiotics 10 days prior to the consultation, without improvement.
Physical and neurological evaluation
Physical evaluation was unremarkable in 5 cats. In one cat (case 3) episodic tachypnea was observed, one cat (case 1) had a reduced body condition score and one cat (case 5) presented with mild dehydration.
Muscle atrophy was not present in any of the cases, but case 5 had painful muscles on palpation. In all but one cat (case 5), the neurological examination revealed severe generalized fatigability in which the cat became tetraparetic after a few steps, with stiffened gait, then collapsed and stayed in sternal recumbency.
Generalized tremors were observed during the effort in cases 2 and 4. After a few minutes rest, the animals were again able to walk a few steps. Two cats (case 3 and case 4) were almost permanently non-ambulatory and stayed in sternal recumbency resting the head on the paws.
Six cats presented with neck ventroflexion (Table 1). Postural reactions were normal in all cases, except for hopping which was reduced in cases 3 and 7, and general proprioception which was slightly decreased on all four limbs in case 3.
The withdrawal reflex was diminished on the thoracic limbs in case 7, and on all four limbs in case 3. Case 7 had a reduced menace response (without visual deficits) and absent palpebral reflex. Ptosis was absent in all cases. Nociception was normal in all cases. The neuroanatomical diagnosis was generalized neuromuscular disease in all cases, most consistent with a disease affecting the neuromuscular junction.
Diagnostic imaging and mediastinal mass exploration
All animals underwent thoracic radiographs. Four cats (cases 1 and 2 for confirmed cases, and the two suspected cases) also had a chest CT scan, and one cat (case 5) had thoracic ultrasonography. A cranial mediastinal mass compatible with thymoma was observed in 5 cats (cases 1, 3, 5, 6 for confirmed cases, and case 7).
Thymoma was confirmed in cases 5 and 7 by histological examination, and in case 1 by cytological examination of a fine needle aspirate.
A megaesophagus was suspected in 2 cats (cases 4 and 7), based on the esophageal dilation on the thoracic radiographs. One cat (case 2) underwent thoracic fluoroscopy, ruling out a megaesophagus. Case 6 underwent a brain MRI and CSF tap because of an initial suspicion of encephalopathy.
The MRI scan performed with a low-field device revealed a focal meningeal mild FLAIR hyperintensity in the falx cerebri, with a moderate contrast enhancement on T1-weighted images after gadoteric acid (DOTAREM, Guerbet, France) administration. The cerebrospinal fluid analysis was otherwise unremarkable.
Clinicopathologic data
Clinicopathologic abnormalities in the 6 confirmed cases included elevated serum CK activity in all but one cat (not evaluated in case 6), ranging from 845 to 23,200 UI/L (reference interval: 0–250 UI/L, Table 2).
Three of the animals presented with mild to moderate dysorexia (cases 1, 4 and 5) at the time of admission, and all animal weights remained stable (except for case 1 in which the body condition score was reduced). Regarding the two suspected cases, case 7 displayed elevated serum CK activity (2553 UI/L), whereas case 8 did not (174 UI/L).
The remaining routine blood work in all the cats was otherwise unremarkable, except in case 5 which presented with neutropenia and thrombocytopenia. In this case, a bone marrow examination revealed significant myeloid hypoplasia, especially in the neutrophil series, and megakaryocytosis, with vacuolized megakaryocytes.
An immune-mediated origin was therefore suspected as the FIV, FeLV, antinuclear antibodies and toxoplasmosis tests were all negative. In the confirmed cases, serum AChR antibody titer ranged from 4.61 to 9.01 nmol/L (reference value <0.3 nmol/L). It was not evaluated in case 7, and normal (0.11 nmol/L) in case 8 (Table 2).
Electrodiagnostics
An electrophysiological examination was performed one day to one week after admission, and at least two weeks after the onset of clinical signs in all cats. Electromyography revealed abnormal spontaneous electrical activity in 5/6 confirmed cases (cases 1, 2, 3, 5 and 6), characterized by fibrillation potentials (all cases) and positive sharp waves (cases 1, 2 and 5). A mild (case 4) to moderate (case 5) prolonged insertional activity was also observed (Table 2).
Abnormal spontaneous activity was marked in case 1, moderate in cases 2, 5 and 6, and mild in case 3 (only in the Mm interossei in the latter case).
Abnormal spontaneous activity in the two suspected cases was mild (case 8, fibrillation potentials in Mm interossei only) to moderate (case 7, fibrillation potentials and positive sharp waves).
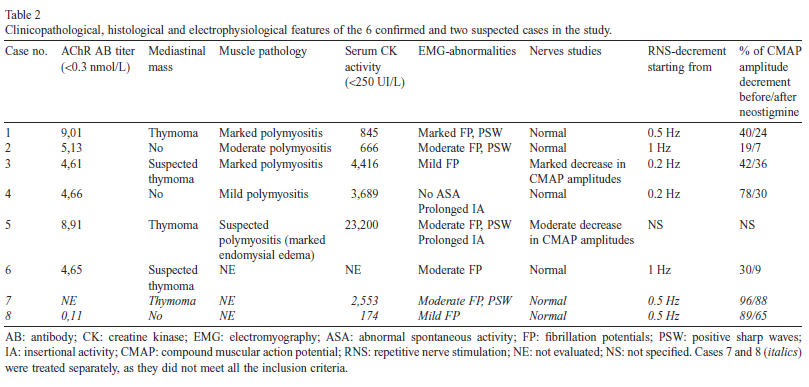
Nerve studies (either motor or sensory) were unremarkable in all but cases 3 and 5. In case 3, CMAP amplitudes were significantly decreased in the ulnar, tibial and peroneal nerves (with values ranging from 1.1 to 9.6 mV regardless of the tested nerve). In case 5, CMAP amplitudes were moderately decreased for the peroneal nerve, ranging between 10.8 and 12.5 mV.
However, in both cases the motor nerve conduction velocities (MNCV) were within normal limits. It should be noted that the nerve studies (either motor or sensory) were also unremarkable in the two suspected cases, which does not support a polyneuropathy in these cats.
On RNS, decrement of the CMAP was observed in all tested cases (even in the two suspected cases), from stimulation frequencies of 0.2 Hz (cases 3 and 4), 0.5 Hz (cases 1), and 1 Hz (case 2 and 6) but it was not specified for case 5. The decrement ranged from 19% to 96% of the initial CMAP amplitude. The decrement was resolved in cases 2 and 6 (7% versus 19% before neostigmine methylsulfate administration, and 9% versus 30% respectively), was strongly improved in cases 1 and 4 (24% versus 40%, and 30% versus 78% respectively) and was less pronounced in case 3 (36% versus 42%), after neostigmine methylsulfate administration.
In the two additional cases (7 and 8), the decrement was observed from 0.5 Hz in both cases, and was mildly (88% versus 96% for case 7) to moderately (65% versus 89% for case 8) improved, after neostigmine methylsulfate administration.
Muscle histology
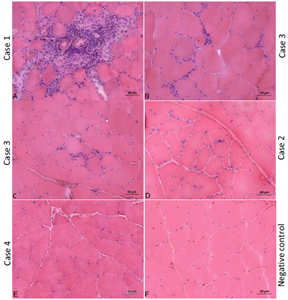 Fig. 1. Histopathology of muscle biopsies, hematoxylin & eosin (HE) staining. (A) Biceps femoris from case 1, (B) biceps femoris from case 3, (C) triceps brachii from case 3; both cases being affected by thymoma. (D) Tibialis cranialis from case 2 (unaffected by thymoma), (E) tibialis cranialis from case 4 (unaffected by thymoma), compared to biceps femoris from a healthy cat (F). Note the clearly apparent inflammatory cell infiltrates in images A, B, C, and to a lesser extent in image D, and the variability in myofiber size and shape in all muscles of MG cats, along with numerous centralized nuclei (Bar = 50 m).
Fig. 1. Histopathology of muscle biopsies, hematoxylin & eosin (HE) staining. (A) Biceps femoris from case 1, (B) biceps femoris from case 3, (C) triceps brachii from case 3; both cases being affected by thymoma. (D) Tibialis cranialis from case 2 (unaffected by thymoma), (E) tibialis cranialis from case 4 (unaffected by thymoma), compared to biceps femoris from a healthy cat (F). Note the clearly apparent inflammatory cell infiltrates in images A, B, C, and to a lesser extent in image D, and the variability in myofiber size and shape in all muscles of MG cats, along with numerous centralized nuclei (Bar = 50 m).
Five animals underwent muscle biopsies (cases 1–5, Table 2).
Cellular infiltrates consistent with a diagnosis of polymyositis were evident in the biceps femoris of cases 1 and 3 (Fig. 1A and 1B) and in the triceps brachii of case 3 (Fig. 1C) on routine histological examination with H&E staining. Both cats presented with a cranial mediastinal mass and elevated serum CK activity.
Muscle biopsies from these two cases showed a moderate to marked variability in myofiber size, with atrophic fibers in both cases having a round shape and of both fiber types. Multifocal areas of mixed mononuclear cell infiltrations having an endomysial and perimysial distribution were present, and scattered necrotic fibers were also observed.
In both cases these findings were most pronounced in the biceps femoris muscles. In the two cases without thymoma, biopsy of the tibialis cranialis revealed mild (case 4, Fig. 1E) to moderate (case 2, Fig. 1D) variability in myofiber size and centralized nuclei with H&E staining, when compared to a negative control (Fig. 1F).
Case 2 also revealed cellular infiltrates in the tibialis cranialis (Fig. 1D), but to a lesser extent compared to cases 1 and 3. Case 5 only showed moderate to marked endomysial interstitial edema of the myofibers in the biceps femoris (not shown).
IHC analysis (Figs. 2–4) of all the cases tested (cases 1, 2, 3 and 4), with or without thymoma, revealed a similar pattern of inflammatory cell infiltrations, but of variable severity. Localization by esterase reaction and α-bgt labeling (not shown) did not reveal any cellular infiltrates in the vicinity of motor end-plates in any of the cases.
Cases 1 and 3 with thymoma showed mild to moderate multifocal and diffuse endomysial and perimysial CD4/CD8 (case 1, Fig. 2B and 2C) and CD3/CD11b (case 3, Fig. 2E and 2H) positive inflammatory cell infiltrates, in addition to the major areas of infiltration observed with H&E staining, when compared to a negative control (Fig. 3I–3L).
These findings were less pronounced for CD4 and CD8 antibodies in case 3 (Fig. 2F and 2G), and for CD3 and CD11b antibodies in case 1 (Fig. 2A and 2D). The two cases unaffected by thymoma (cases 2 and 4) also showed moderate diffuse endomysial infiltrates of CD4 positive cells in the tibialis cranialis muscle of case 2 (Fig. 3B), and focal less pronounced endomysial infiltrates of CD11b (Fig. 3H) and CD4 (Fig. 3F) positive cells in the tibialis cranialis muscle of case 4, when compared to the negative control (Fig. 3I–3L).
In addition, mild focal CD3 and CD11b positive cells were also observed in case 2 (Fig. 3A and 3D). A CD8 coloration uptake around the myofibers was also observed in case 2 (Fig. 3C). IHC analysis with antibodies against MHC-I and C5b-9 (Fig. 4) was positive in all tested cases. A diffuse and sustained sarcolemmal overexpression of MHC-I and C5b-9 was observed in biceps femoris of case 1, surrounding the major areas of necrosis (Fig. 4A and 4B).
This was also obvious but less pronounced in biceps femoris of case 3 (Fig. 4E and 4F).
Case 2 (without thymoma) also displayed MHC-I and C5b-9 diffuse positive areas in tibialis cranialis (Fig. 4C and 4D). Tibialis cranialis of case 4 showed the minimal overexpression of MHC-I and C5b-9 (Fig. 4G and 4H), but was nevertheless positive compared to a muscle from a healthy cat (Fig. 4I and 4J).
Selected histological, immunohistological and biochemical markers allowing diagnosis of PM are summarized in Table 3.
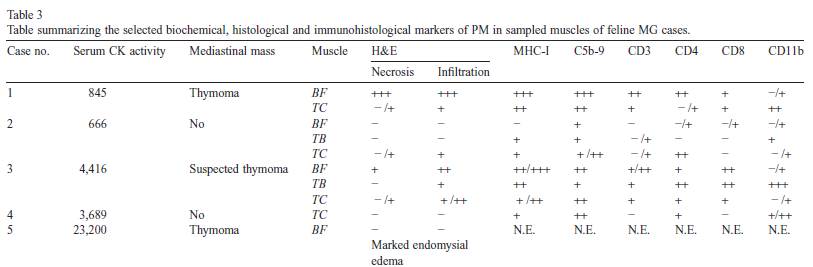 Table 3 : PM: polymyositis; N.E.: not evaluated; BF: biceps femorii; TB: triceps brachii; TC: tibialis cranialis; H&E: hematoxylin & eosin; MHC-I: major histocompatibility complex class 1. Reference range for CK: < 250 UI/L. Key notation: − : no abnormality, +: mild, rare and sparse inflammatory cells infiltrates/sarcolemmal depositions; ++: moderate, several sustained multifocal inflammatory cell infiltrates/sarcolemmal depositions; +++: marked, diffuse extensive inflammatory cell infiltrates/sarcolemmal depositions.
Table 3 : PM: polymyositis; N.E.: not evaluated; BF: biceps femorii; TB: triceps brachii; TC: tibialis cranialis; H&E: hematoxylin & eosin; MHC-I: major histocompatibility complex class 1. Reference range for CK: < 250 UI/L. Key notation: − : no abnormality, +: mild, rare and sparse inflammatory cells infiltrates/sarcolemmal depositions; ++: moderate, several sustained multifocal inflammatory cell infiltrates/sarcolemmal depositions; +++: marked, diffuse extensive inflammatory cell infiltrates/sarcolemmal depositions.
Treatment and follow-up
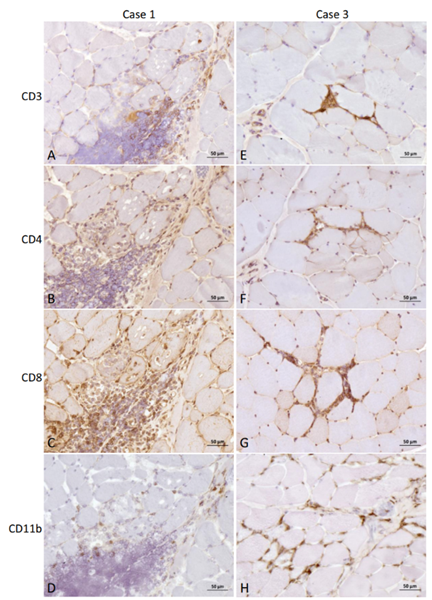 Fig. 2. Immunohistochemistry of muscle biopsies from case 1 and case 3, both affected by thymoma. Anti-CD3 (A), -CD4 (B), -CD8 (C), and -CD11b (D) antibody of biceps femoris from case 1, and anti-CD3 (E), -CD4 (F), -CD8 (G) and -CD11b (H) antibody of biceps femoris from case 3. Note the diffuse CD4 and CD8 (case 1, B and C), and CD3 and CD11b (case 3, E and H) positive cell infiltration, in addition to the major infiltrates observed with H&E staining (Bar = 50 m).
Fig. 2. Immunohistochemistry of muscle biopsies from case 1 and case 3, both affected by thymoma. Anti-CD3 (A), -CD4 (B), -CD8 (C), and -CD11b (D) antibody of biceps femoris from case 1, and anti-CD3 (E), -CD4 (F), -CD8 (G) and -CD11b (H) antibody of biceps femoris from case 3. Note the diffuse CD4 and CD8 (case 1, B and C), and CD3 and CD11b (case 3, E and H) positive cell infiltration, in addition to the major infiltrates observed with H&E staining (Bar = 50 m).
All cats (with the exception of case 5) received an anticholinesterase drug following the diagnosis of MG (Table 1).
Cases 1 and 2 were treated with an injectable form of neostigmine methylsulfate (Prostigmine, Meda Pharma, France): Case 1 received 0.05 mg/kg q8h intramuscularly, and case 2 received 0.04 mg/kg q8h subcutaneously, which was then gradually tapered. Cases 2, 3, 4 and 6 were given pyridostigmine bromide tablets (Mestinon, Meda Pharma, France).
Case 2 initially received 1 mg/kg q8h (switched to an injectable form after poor response), case 3 received 0.25 mg/kg q12h, case 4 received 0.35 mg/kg q12h (gradually increased to 0.75 mg/kg q8-6h two days after diagnosis) and case 6 received 1.3 mg/kg q12h. Both suspected cases were treated with the tablet form of ambenonium chloride (Mytelase, Sanofi Aventis, France).
Case 7 received 1.6 mg/kg q12h, doubled one week after diagnosis due to poor response, and case 8 received 0.5 mg/kg q8h (with attempts to taper the dose). Four cases received prednisolone (either Microsolone, Merial, France, or Dermipred, Sogeval, France): case 5 had prednisolone alone (starting from 2.4 mg/kg/d, then tapering of the dose), case 7 in combination with ambenonium chloride (2 mg/kg/d), case 6 in combination with pyridostigmine bromide (2 mg/kg/d, then tapered) and case 2 in which prednisolone was added in a second time (1 mg/kg/d, then tapered) after an anticholinesterase drug. Note that none of the cats (including the two suspected cases) experienced adverse effects related to the anticholinesterase therapy. Two cats (Cases 1 and 4) died soon after admission from an unexplained cause: Case 1 died under anesthesia for the thoracic CT (conducted to explore the cranial mediastinal mass), and case 4 died unexpectedly during hospitalization (the owner declined autopsy).
Case 3 was euthanized one week after admission due to poor response to therapy.
Case 2 (not affected by thymoma) first received oral pyridostigmine bromide which provided clinical improvement for a few weeks, but clinical signs reappeared one month after diagnosis. Therefore, prednisolone was added at a dose of 1 mg/kg/d, and pyridostigmine bromide was replaced by the injectable form of neostigmine methylsulfate at 0.04 mg/kg q8h SC, with both doses tapered gradually.
Clinical signs resolved progressively over a period of one year to reach clinical and serological remission (follow-up AChR antibody titer was 0.03 nmol/L, <0.3 nmol/L, after 5.13 nmol/L initially). The cat was doing well one year after the treatment was discontinued.
Case 5 underwent a thoracotomy to remove the thymoma. A gradual improvement was observed, but the cat relapsed two years after surgery. A corticosteroid treatment was re-initiated with clinical remission two years later (i.e. four years after diagnosis, no follow-up AChR antibody titer available).
Case 6 was doing well eight months after the diagnosis, and was still receiving prednisolone and pyridostigmine bromide at the time of writing. Both the suspected cases responded well to treatment.
Case 7 died for unknown reasons 6 months after the diagnosis, while still receiving prednisolone and ambenonium chloride.
Case 8 was doing well five years after the diagnosis and was still receiving ambenonium chloride at the time of writing. However, the owner reports a recurrence of clinical signs when he attempts to reduce the dose or frequency of ambenonium chloride, which is an additional element consistent with MG in this suspected case.
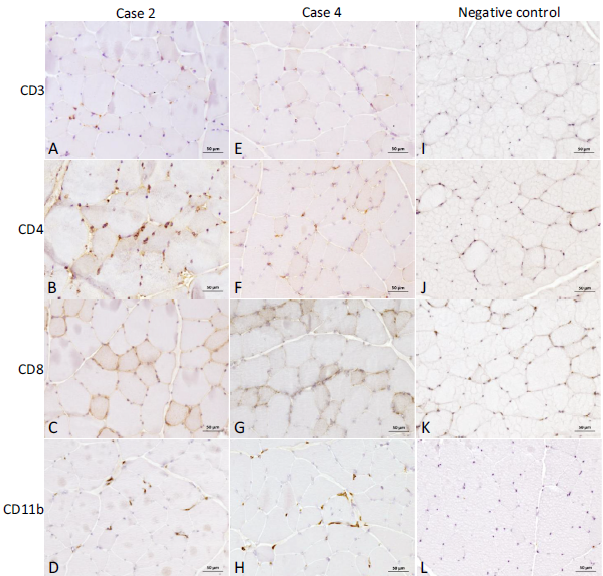 Fig. 3. Immunohistochemistry of muscle biopsies from case 2 and case 4, unaffected by thymoma, compared to a healthy cat. Anti-CD3 (A), -CD4 (B), -CD8 (C) and -CD11b (D) antibody of tibialis cranialis from case 2, anti-CD3 (E), -CD4 (F), -CD8 (G) and -CD11b (H) antibody of tibialis cranialis from case 4, compared to similarly stained muscles from a negative control (healthy cat, images I, J, K and L). Note the mild CD3 (A) to moderate diffuse CD4 (B) and CD11b (D) positive cell infiltration in case 2, and the mild CD4 (F) to moderate CD11b (H) positive cell infiltration in case 4, compared to the negative control. Anti-CD8 antibody coloration was however very similar to the negative control in both cases (C, G and K) (Bar = 50 m)
Fig. 3. Immunohistochemistry of muscle biopsies from case 2 and case 4, unaffected by thymoma, compared to a healthy cat. Anti-CD3 (A), -CD4 (B), -CD8 (C) and -CD11b (D) antibody of tibialis cranialis from case 2, anti-CD3 (E), -CD4 (F), -CD8 (G) and -CD11b (H) antibody of tibialis cranialis from case 4, compared to similarly stained muscles from a negative control (healthy cat, images I, J, K and L). Note the mild CD3 (A) to moderate diffuse CD4 (B) and CD11b (D) positive cell infiltration in case 2, and the mild CD4 (F) to moderate CD11b (H) positive cell infiltration in case 4, compared to the negative control. Anti-CD8 antibody coloration was however very similar to the negative control in both cases (C, G and K) (Bar = 50 m)