Acquired canaliculocele with intranasal extension in a dog
Introduction
Noninflammatory swellings ventromedial to the eye are uncommon in dogs. Possible origins have been described, including epidermoid cysts,1–3 maxillary bone epithelial cysts,4 and other cysts, mucocele and dilatation of the periorbital glandular and duct tissues such as the zygomatic salivary gland,5 the lacrimal gland,5–7 the third eyelid lacrimal gland,5 the lacrimal excretory system,8–10 the conjunctival epithelium, and the nasal or frontal sinus mucosa.5
Congenital conditions affecting the lacrimal drainage system include abnormalities such as atresia of the lacrimal punctum,9 ectopic lacrimal punctum and canaliculus, atresia of the lacrimal canaliculus and lacrimal sac,9 dacryops, 6,11 and canaliculops.12
Acquired nasolacrimal duct obstructions are common in adult dogs and are often associated with dacryocystitis due to foreign-body obstruction or infection.9,12–14 Less frequently, extraluminal lesions may be neoplastic,9,15,16 cystic,17–20 traumatic (maxillary and lacrimal bone fractures), 8 or inflammatory in origin19,21 when the nasal sinuses may impinge on the nasolacrimal duct. Canalicular cysts or canaliculoceles may result from the progression of congenital canalicular ectasia.9 Epiphora is frequently the major clinical sign with concurrent excoriation and conjunctivitis.9,10

Figure 1. Photograph of a 6-year-old Jack Russell Terrier neutered female dog. Note the mass around the medial canthus of the right eye of 5-month duration. No ocular discharge was observed.
Minimally invasive surgery of a lacrimal drainage system cyst with endonasal extension is poorly documented in dogs.10
In this report, we describe an unusual occurrence of a canaliculocele with intranasal extension and destruction of the adjacent maxilla. Plain radiography was used to identify anatomical abnormalities in the periorbital region. However, computerized tomography (CT) provided more precise morphological information concerning the lesion. We performed endoscopic endonasal marsupialization resulting in recovery of this dog. A definitive diagnosis was only achieved with histological categorization following the surgery. No recurrence was observed during follow-up.
Case report
Case history
A 6-year-old female neutered Jack Russell Terrier was referred to the Centre Hospitalier Vétérinaire des Cordeliers for a slowly enlarging mass around the medial canthus of the right eye of 5-month duration (Fig. 1). Intermittent serous ocular discharge had been noted, with nasal stridor and more recently episodic sneezing. The referring veterinarian had diagnosed an abscess of the root of the fourth premolar and removed the carnassial tooth and the two upper right molars 4 months earlier. Systemic therapy was used in combination with surgery and consisted of the fluoroquinolone antibiotic marbofloxacin (4 mg/kg orally once daily administered for 15 days, Marbocyl® 20 mg; Vetoquinol, Lure, France) and the nonsteroidal anti-inflammatory agent tolfenamic acid (4 mg/kg orally once daily administered for 3 days, Tolfédine® 20 mg; Vetoquinol). No improvement was noted. Aspiration of the mass, 2 months before referral, produced watery fluid and size reduction, but the mass enlarged again within a few days.
The swelling was not associated with any discomfort. There was no history of prior ocular or periocular inflammation or trauma.
Medical treatment was discontinued for 3 months.
Clinical findings
There was no evidence of systemic disease during the general physical examination. Complete blood count and routine biochemistry were within normal limits.
Physical examination revealed a 3-cm-diameter, firm, round, immobile subcutaneous mass beneath the medial canthus of the right eye, overlying the maxillary bone. Palpation of the maxillary bone revealed a defect in the bone below the area of soft tissue swelling. Upon applying digital pressure to the mass, there was no fluid reflux through the lacrimal puncta, but some movements of the eye were present. Inflammatory signs such as erythema were not found over the swelling. Palpation of regional lymph nodes revealed no abnormalities.
Applanation tonometry revealed intraocular pressures of 14 mmHg in the right eye (OD) and 13 mmHg in left eye (OS) (Tono-Pen® XL; Mentor Worldwide LLC, Santa Barbara, CA, USA). Values for the Schirmer tear test (Laboratoire TVM, Lempdes, France) were 16 and 12 mm/min OD and OS, respectively. A normal menace response and palpebral reflex were present in both eyes, and the direct and consensual pupillary light reflexes were also normal. Slit-lamp microscopy (SL-15 Portable Slit-Lamp Biomicroscope; Kowa Co. Ltd, Tokyo, Japan) and fundoscopy (Omega® 500 LED indirect ophthalmoscope; Heine Optotechnik, Herrsching, Germany and Volk Pan Retinal 22 diopter indirect ophthalmoscopy lens; Nidek S.A., Saint-Priest, France) were completed, and no abnormalities were noted. Fluorescein dye (Fluorescéine® 0.5% unidose eye drops; Laboratoire TVM) applied topically to each eye appeared in the left nares within 3 min, but did not appear in the right after 20 min.
A 24-gauge nasolacrimal metal cannula preplaced on a 5-mL syringe containing saline solution, and a drop of topical anesthetic (Tetracaine® 1% eye drops, Laboratoire TVM) was applied to the conjunctiva. The canaliculi of the right eye were cannulated, and a small volume of saline solution injected while observing the other lacrimal punctum. Abnormal resistance and relative distension of the mass was noticed during attempts to flush saline through the cannulated superior or inferior canaliculus. It was not possible to flush saline from the superior to the inferior punctae. No saline solution was detected in the right nares.
Percutaneous fine needle aspiration of the mass was performed using a syringe attached to a 22 G needle. The mass yielded a clear, colorless, watery fluid. Laboratory analysis revealed a specific gravity of 1008, a protein content of 0.9 g/L and no cells. Bacteriology on the fluid was negative for aerobic and anaerobic growth. Fungal culture was negative.
Pre-operative imaging
The dog was premedicated with an intramuscular combination of acepromazine (0.03 mg/kg, Calmivet®; Vétoquinol, Lure, France) and morphine (0.4 mg/kg, Morphine Aguettant®; Aguettant, Lyon, France) 30 min before induction.
Anesthesia was induced with alfaxalone (2 mg/kg intravenously, Alfaxan®; Vetoquinol) to effect for tracheal intubation and isoflurane and oxygen maintenance (Vetflurane®; Virbac, Carros, France).
Evaluation of the swollen area by B-mode ultrasonography revealed an anechoic cavitary structure, lined by a thin wall. Posterior acoustic enhancement was present (My-Lab®One; 15–22 MHz linear array transducer; Esaote France, Saint-Germain-en-Laye, France).
Dorsoventral and lateral radiographs of the skull were obtained and revealed a 10-mm-wide lucent area dorsal to PM3 and PM4 suggesting lysis of the maxillary bone and underlying turbinates (High Frequency X-ray Generator Sedecal compact 320®; Sedecal, Madrid, Spain) The bony limits of the lytic area were smooth and well delineated, consistent either with pressure lysis due to a slow-growing mass or a very aggressive lytic lesion.
Dacryocystorhinography was performed on the right side by catheterizing the right superior lacrimal duct with a 24-gauge nasolacrimal metal cannula and injecting 4 mL of a 1:1 dilution of iohexol (Omnipaque 300®, GE Healthcare SA, Velizy, France, 300 mg iodine/mL) and isotonic saline solution. Dorsoventral and lateral radiographs were obtained. Contrast medium accumulation was visible in the area of the right maxillary recess; filling of the proximal part of the nasolacrimal duct could not be achieved (Fig. 2).
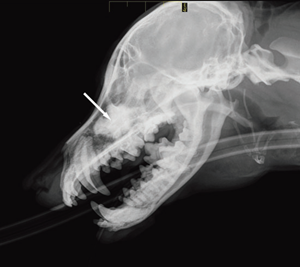
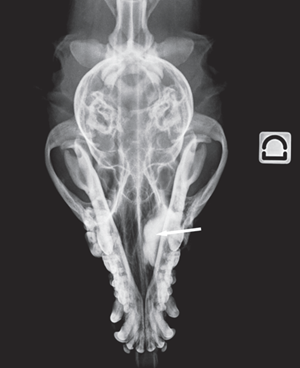 Figure 2 a Lateral view Figure 2 b Dorsoventral view
Fig. 2 a,b : Views of the dacryocystorhinogram of the right nasolacrimal system in a 6-yearold Jack Russell Terrier neutered female dog. Contrast material accumulated in a cystic cavity (arrows) within the right maxillary sinus but did not extend into the nasolacrimal duct distal to the cystic cavity.
Figure 2 a Lateral view Figure 2 b Dorsoventral view
Fig. 2 a,b : Views of the dacryocystorhinogram of the right nasolacrimal system in a 6-yearold Jack Russell Terrier neutered female dog. Contrast material accumulated in a cystic cavity (arrows) within the right maxillary sinus but did not extend into the nasolacrimal duct distal to the cystic cavity.
For further assessment, the dog underwent computerized tomography (CT) that extended from the lower aspect of the orbits through the nasal cavity. The computerized tomography studies were carried out using a 16-row multislice CT scanner (Philips Brilliance Mx 8000 IDT 16 CT Scanner, Philips Medical Systems, Hamburg, Germany). Two-millimeter computerized tomographic images (100 mA, 120 kV) were acquired, with both bone and soft tissue algorithms, from the orbital region to the rostral part of the nasal cavity to cover the length of the lacrimal duct. A 10-mm-wide bone defect was observed in the dorsolateral part of the right maxillary bone, rostral to the orbital cavity, at the level of the P3 to M1 tooth roots (see image). Its borders were smooth, and no proliferation could be seen. A soft tissue density, 17 mm wide and 20 mm long, occupied the ventrolateral part of the nasal cavity at that level, slightly protruding through the bone defect and severely reducing the nasal cavity diameter. After IV iodinated contrast injection (ioxitalamate 700 mg/kg IV, Omnipaque 350®, Guerbet, Roissy CDG, France), a thin rim enhancement was observed around this mass, suggesting the presence of a thin wall (Fig. 3).
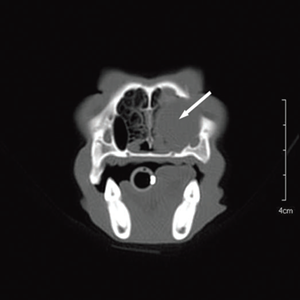
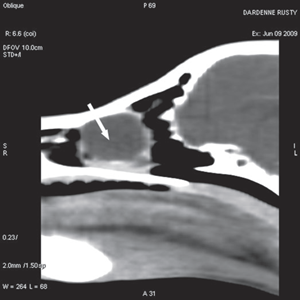
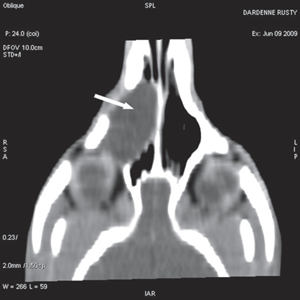 Figure 3a Transverse view Figure 3b Sagittal view Figure 3c Coronal view
Figure 3a Transverse view Figure 3b Sagittal view Figure 3c Coronal view
Fig. 3 a,b,c : computerized tomography (CT) imaging at the orbit and pterygopalatine fossa level. A well-circumscribed cystic structure (arrows) can be identified extending into the orbit and nasal cavity.
Three-dimensional reconstruction of CT image clearly demonstrated a bone defect of the maxillary, lacrimal, and frontal bones (Fig. 4).
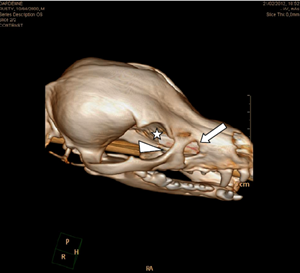 Figure 4 Three-dimensional volume rendering reconstruction of the computerized tomography (CT) imaging. It demonstrated the defect in the right maxillary (arrow), lacrimal (arrowhead), and frontal (asterisk) bones more clearly.
Figure 4 Three-dimensional volume rendering reconstruction of the computerized tomography (CT) imaging. It demonstrated the defect in the right maxillary (arrow), lacrimal (arrowhead), and frontal (asterisk) bones more clearly.
The diagnostic imaging results were consistent with lacrimal duct dilatation, suggesting a lacrimal obstruction either due to endoluminal filling or parietal stenosis, because no periductal mass could be demonstrated. Given the extent of the mass, its effect on breathing and its cavitary nature, an endoscopic treatment with incision, decompression, and marsupialization of the endonasal lesion was recommended and scheduled.
Surgery
An anterograde approach was performed via the right nasal chamber. A rigid endoscope (Karl Storz Endoscopie France SAS, Guyancourt, France) with a diameter of 2.7 mm with a 30° angle and a length of 19 cm was used, coupled to a three-CCD camera (Tricam®; Karl Storz Endoscopie France SAS). This imaging system uses three separate charge-coupled devices (CCD), each one taking a separate measurement of the primary colors, red, green, or blue light.
The endoscope was inserted inside a 4-mm-diameter sheath with two Luer-lock taps for irrigating with fluids and aspiration of secretions, with a large (1.65 mm) instrument channel for flexible biopsy and grasping forceps. Continuous irrigation was provided by an arthroscopic irrigation set (Arthropump® plus; Karl Storz Endoscopie France SAS). This irrigation system combines fluid supply and suction in one unit and thus provides a consistently clear field of view.
Nasal endoscopy showed a mass in the dorsal nasal meatus. To prevent any secondary scarring, all the visible rostral cavitary mass wall was resected, thus establishing drainage. Opening of the mass was performed with flexible endoscopic grasping forceps (Type 67761 T®; Karl Storz Endoscopie France SAS) introduced through the operating channel of the optical sheath. The opening was then enlarged with larger rigid grasping forceps (Blakesley® Nasal Forceps type 456500; Karl Storz Endoscopie France SAS) introduced parallel to the rhinoscope under visual guidance. Only the cavitary mass wall surface in contact with and firmly attached to the adjacent osseous surface was left intact (Fig. 5).
Following marsupialization, the lacrimal drainage system was irrigated via the nasal chamber. No stent was placed. The superior canaliculus was cannulated, and the 24-gauge metal probe was easily visualized in the cavity of the mass (Fig. 5). The inferior canaliculus was also cannulated. The nasolacrimal probe was not visible within the cavity, but movements of the probe produced visible movements under the mass wall.
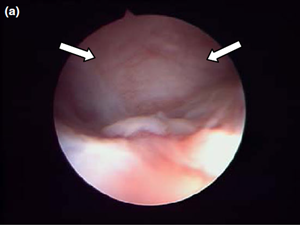
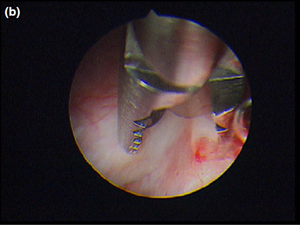
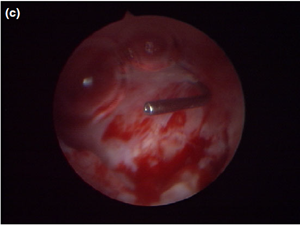
Figure 5 a Endoscopic endonasal view of the right dorsal meatus, showing the cyst filling the meatus before marsupialization (arrows).
Figure 5 b The cyst wall was opened using a straight Blakesley forceps.
Figure 5 c The appearance of the dorsal meatus after marsupialization and excision of the canaliculocele, with the nasolacrimal duct probe still in place.
Postoperatively, the dog was treated with oral cephalexin antibiotic (15 mg/kg every 12 h, Rilexine®; Virbac) and the nonsteroidal anti-inflammatory drug carprofen (4.4 mg/kg once daily, Rimadyl®; Pfizer Santé animale, Paris, France) for 2 weeks.
One year postoperatively, the animal remains asymptomatic with no recurrence of the cyst found after radiographic examination and a dacryocystorhinogram as previously described. The contrast medium flowed freely through the nasal cavity (Fig. 6).
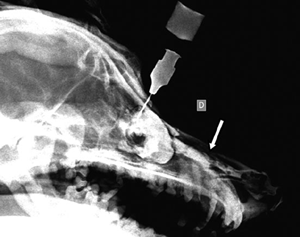 Figure 6 One year postoperatively, the contrast medium flowed freely through the nasal cavity (arrow).
Figure 6 One year postoperatively, the contrast medium flowed freely through the nasal cavity (arrow).
Histology
Histology confirmed a cystic lesion with a lining epithelium composed mostly of multilayered or pseudostratified cuboidal cells with underlying fibrosis (Fig. 7). No goblet cells or cilia could be detected. Calcification of the cyst wall, probably secondary to chronic low-grade inflammation, was noted and has been reported elsewhere.13 The histological diagnosis was a non-neoplastic cyst. Immunohistological staining for smooth muscle actin and desmin were performed and were both negative (Fig. 8).
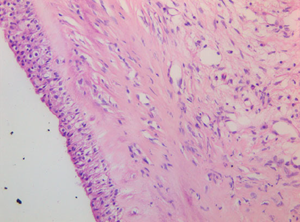 Figure 7 Cyst wall consisting of multilayered cuboidal cells with underlying fibrous tissue. 409 objective, HE stain.
Figure 7 Cyst wall consisting of multilayered cuboidal cells with underlying fibrous tissue. 409 objective, HE stain.
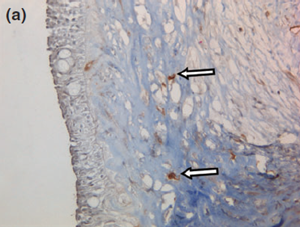
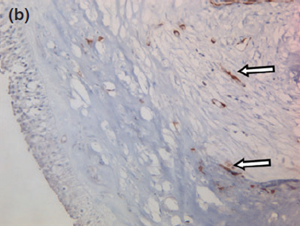 Figure 8 a Representative photographs of the negative immunohistochemical staining for (a) smooth muscle actin and (b) desmin confirmed the absence of myoepithelial cells under the cyst wall.
Figure 8 a Representative photographs of the negative immunohistochemical staining for (a) smooth muscle actin and (b) desmin confirmed the absence of myoepithelial cells under the cyst wall.
Figure 8 b The blood vessels are surrounded by numerous myoepithelial cells, serving as a positive control (arrows). 409 objective, counterstained with hematoxylin and eosin.